【1st.Dec.】Selective, Three-component Dicarbofunctionalization of Unactivated Alkenes via Nickel Catalysis
日期:2020-12-01
阅读:909
报告人:储玲玲 研究员,博士生导师,东华大学
时间:12月1日下午1:30-5:00
地点:化学B楼410会议室
邀请人:张兆国教授
个人简历

获得奖励和荣誉
2020 上海市“科技创新行动计划”启明星项目
2020 上海市第十一届巾帼创新新秀奖及上海市三八红旗手
2019 国家自然科学二等奖 (排名第二)
2017 上海市自然科学一等奖 (排名第二)
2017 上海市青年科技英才扬帆计划
2012 中科院优秀博士论文
社会兼职:
1. Science Bulletin 青年编委
2. 中国化学会青年委员会委员
研究兴趣与方向
光促自由基化学,镍催化选择性转化,有机含氟材料
近三年代表性成果
1. Guo, L.;# Yuan, M.;# Zhang,Y.; Wang, F.; † Zhu, S. Gutierrez, O.,* Chu, L.*, “Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins Through Nickel-Catalyzed Cross-Electrophile Coupling”, J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.0c08823.
2. Yang, J.;# Zhu, S.;# Wang, F.; Qing, F,; Chu, L.*, “Silver-Enabled General Radical Difluoromethylation Reaction with TMSCF2H”, Angew. Chem. Int. Ed. 2020, DOI: 10.1002/anie.202014587.
3. Tu, H.-Y.;# Wang, F.;# Huo, L.; Li, Y.; Zhu, S.; Zhao, X.; Li, H.; Qing, F.-L.; Chu, L.*, “Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins Through Nickel-Catalyzed Cross-Electrophile Coupling”, J. Am. Chem. Soc. 2020, 142 (21), 9604–9611.
4. Song, F.;# Wang, F.;# Guo, L.; Feng, X.-L.; Zhang, Y.-Y.; Chu, L.*, “Visible-Light-Enabled Stereodivergent Synthesis of (E)- and (Z)-1,4-Dienes via Photoredox/Nickel Dual Catalysis”, Angew. Chem. Int. Ed. 2020, 59 (1), 177-181. (Hot Paper)
5. Li, H;# Guo, L.;# Feng, X.; Huo, L.; Zhu, S.; Chu, L.*, “Enantioselective Three-Component Fluoroalkylarylation of Unactivated Olefins Through Nickel-Catalyzed Cross-Electrophile Coupling”, Chem. Sci. 2020, 11, 4904-4910.
6. Guo, L.#; Tu, H.-Y. #; Zhu, S.; Chu, L.*, “Selective, Intermolecular Alkylarylation of Alkenes via Photoredox/Nickel Dual Catalysis”, Org. Lett. 2019, 21, 4771-4776.
7. Zhu, S.; Qin, J.; Wang, F.; Li, H.; Chu, L.*, “Photoredox-catalyzed branch-selective pyridylation of alkenes for the expedient synthesis of Triprolidine”, Nat. Commun. 2019, 10, 749.
8. Chen, D.; Xu, L.; Long, T.; Zhu, S.; Yang, J.; Chu, L.*, “Metal-free, intermolecular carbopyridylation of alkenes via visible-light-induced reductive radical coupling”, Chem. Sci. 2018, 9, 9012-9017.
9. Guo, L.; Song, F.; Zhu, S.; Li, H.; Chu, L.*, “syn-Selective Alkylarylation of Terminal Alkynes via the Combination of Photoredox and Nickel Catalysis” Nat. Commun., 2018, 9, 4543.
10. Zhao, X.#; Tu, H.-Y.#; Guo, L.; Zhu, S.; Qing F.-L.; Chu, L.*, “Intermolecular selective carboacylation of alkenes via nickel-catalyzed reductive radical relay” Nat. Commun., 2018, 9, 3488.
报告摘要:
Selective, Three-component Dicarbofunctionalization of Unactivated Alkenes via Nickel Catalysis
Lingling Chu (储玲玲)
1Center for Advanced Low-dimension Materials, Donghua University, Shanghai, 201610 E-mail: lingling.chu1@dhu.edu.cn
Transition metal-catalyzed dicarbofunctionalization of alkenes has been proven as a powerful strategy to the rapid generation of molecular complexity by simultaneously forging two vicinal sp3 C–C bonds from abundant building blocks in one single operation; however, selective control, particularly with enantioselective control, of the newly formed stereogenic centers in three-component assembly mode, remains a formidable challenge. Utilizing a nickel-catalyzed radical cascade strategy, we have developed a series of intermolecular, three-component dicarbofunctionalization of unactivated alkenes with excellent selectivity under mild conditions, allowing for facile excess to a wide range of highly functionalized fluoroalkyl-containing molecules from simple starting materials.
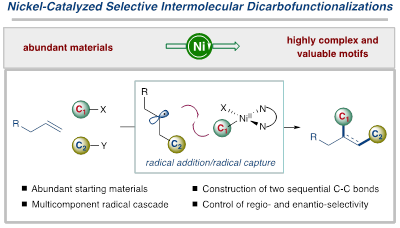
Fig. 1. Ni-catalyzed three-component dicarbofunctionalization reactions
References:
[1] Guo, L.;# Yuan, M.;# Zhang,Y.; Wang, F.; † Zhu, S. Gutierrez, O.,* Chu, L.*, “J. Am. Chem. Soc. 2020, DOI: 10.1021/jacs.0c08823.
[2] Tu, H.-Y.;#, Wang, F.; # Huo, L.; Li, Y.; Zhao, X.; Li, H.; Qing F.-L.; Chu, L.*, J. Am. Chem. Soc. 2020, 142, 9604.
[3] Song, F.;# Wang, F.;# Guo, L.; Feng, X.-L.; Zhang, Y.-Y.; Chu, L.*, Angew. Chem. Int. Ed. 2020, 59, 177-181.
[4] Li, H.;# Guo, L.;# Feng, X.-L.; Huo, L.-P.; Zhu, S.-Q.;; Chu, L.*, Chem. Sci. 2020, 11, 4904-4910.
[5] Guo, L.; # Tu, H.-Y. #; Zhu, S.; Chu, L.*, Org. Lett. 2019, 21, 4771.
[6] Guo, L.; Song, F.; Zhu, S.; Li, H.; Chu, L.*, Nat. Commun., 2018, 9, 4543.
[7] Zhao, X.#; Tu, H.-Y.#; Guo, L.; Zhu, S.; Qing F.-L.; Chu, L.*, Nat. Commun., 2018, 9, 3488.



